IBD and IBS Test
Gut Microbiota test for IBD & IBS
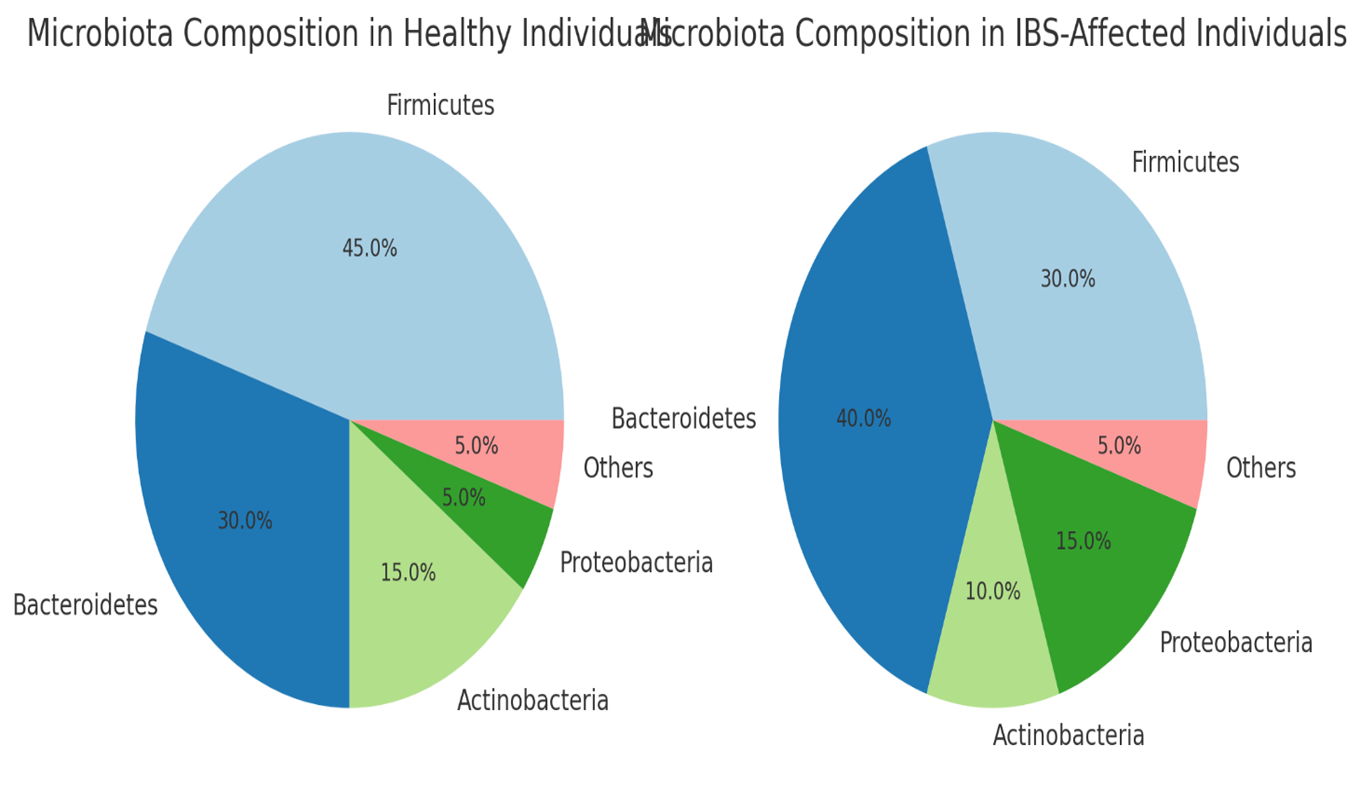
The pie charts illustrate the differences in microbiota composition between healthy individuals (L)and those affected by IBS (R):
- Healthy Individuals:
-
- Higher proportions of Firmicutes (45%) and Actinobacteria (15%), both of which are important for maintaining gut health and producing beneficial metabolites like short-chain fatty acids (SCFAs).
- A balanced representation of Bacteroidetes (30%) and lower levels of Proteobacteria (5%), indicating a healthy gut environment with limited pathogenic bacteria.
- IBS-Affected Individuals:
-
- Reduced Firmicutes (30%) and Actinobacteria (10%), suggesting a lower presence of beneficial bacteria that protect the gut lining and reduce inflammation.
Increased Bacteroidetes (40%) and Proteobacteria (15%), which could contribute to dysbiosis, inflammation, and symptoms like bloating and pain in IBS patients
Key Gut Microbial Species Involved in IBS
- Firmicutes:
- Characteristics: This is one of the dominant phyla in the human gut, composed of many Gram-positive bacteria.
- Role in IBS:
- Studies have shown that people with IBS, especially those with diarrhea-predominant IBS (IBS-D), often have a lower abundance of certain beneficial Firmicutes like Faecalibacterium prausnitzii, which is known for its anti-inflammatory properties and production of short-chain fatty acids (SCFAs), particularly butyrate.
- Conversely, there may be an overrepresentation of other Firmicutes that are less beneficial or potentially harmful, such as some Clostridium species, which could contribute to increased gas production and altered gut motility.
- Bacteroidetes:
- Characteristics: Another major phylum of Gram-negative bacteria, typically involved in carbohydrate fermentation and SCFA production.
- Role in IBS:
- The overall abundance of Bacteroidetes in IBS patients can be either increased or decreased, depending on the subtype of IBS (constipation-predominant IBS-C vs. diarrhea-predominant IBS-D).
- An imbalance in this phylum is thought to impact mucosal integrity and inflammatory responses. For example, higher levels of certain Bacteroides species may contribute to the increased permeability of the gut lining, leading to symptoms like pain and bloating.
- Proteobacteria:
- Characteristics: A diverse phylum that includes many Gram-negative bacteria, some of which are pathogenic (e.g., Escherichia coli).
- Role in IBS:
- An increased abundance of Proteobacteria is often observed in IBS patients, particularly in those with IBS-D. This phylum includes potential pathogens like Escherichia coli and Klebsiella, which can cause intestinal inflammation, gas production, and altered motility.
- A higher presence of Proteobacteria is also linked to increased gut permeability (“leaky gut”) and immune activation, both of which are features commonly observed in IBS.
- Actinobacteria:
- Characteristics: This phylum includes many Gram-positive bacteria, such as Bifidobacterium species, which are generally considered beneficial.
- Role in IBS:
- In IBS, there is often a reduction in the abundance of Bifidobacterium, which plays a crucial role in maintaining gut health by producing SCFAs and inhibiting the growth of pathogens.
- A reduced abundance of Actinobacteria, particularly Bifidobacterium, may contribute to symptoms like pain, bloating, and irregular bowel movements due to the decreased production of anti-inflammatory metabolites.
- Verrucomicrobia:
- Characteristics: A relatively minor but significant phylum, which includes species like Akkermansia muciniphila.
- Role in IBS:
- Akkermansia muciniphila is known for its role in maintaining the gut lining and is often reduced in IBS patients, especially in those with more severe symptoms. Its reduction can lead to compromised mucosal integrity and increased gut permeability, contributing to IBS symptoms.
Summary of Dysbiosis Patterns in IBS
- Decreased: Firmicutes (specifically beneficial species like Faecalibacterium prausnitzii), Bifidobacterium (from Actinobacteria), and Akkermansia muciniphila (from Verrucomicrobia).
- Increased: Certain Proteobacteria (e.g., Escherichia coli), specific Firmicutes species (e.g., some Clostridium species), and variable changes in Bacteroidetes depending on IBS subtype.
Novelgene Biomarkers:
Blautia: Changes in the abundance of Blautia are being considered as potential biomarkers for gut health and disease states. Probiotics and dietary interventions are also being explored to modulate Blautia levels for therapeutic purposes.
Key Characteristics of Blautia:
- Morphology: Blautia species are non-motile, rod-shaped bacteria that form chains or pairs. They are obligate anaerobes, meaning they thrive in environments devoid of oxygen.
- Metabolism: These bacteria primarily produce short-chain fatty acids (SCFAs), particularly acetic acid, which is important for maintaining gut homeostasis, energy metabolism, and anti-inflammatory processes.
- Role in the Gut Microbiome:
-
- Health Benefits: Blautia contributes to gut health by inhibiting pathogenic bacteria, supporting immune function, and maintaining intestinal barrier integrity. It is also involved in fermenting dietary fibers and polysaccharides.
- Association with Diseases: Alterations in Blautia abundance have been linked to various conditions, such as obesity, type 2 diabetes, inflammatory bowel disease (IBD), and neurological disorders, including autism and depression.
Key Metabolites Affected by IBS and IBD
In both irritable bowel syndrome (IBS) and Inflammatory Bowel Disease (IBD), the altered gut microbiota leads to changes in the production of various metabolites, which can significantly affect the gastrointestinal environment and contribute to symptoms and disease progression.
- Short-Chain Fatty Acids (SCFAs)
- Metabolites: Acetate, Propionate, Butyrate
- Role in IBS and IBD:
- SCFAs are produced by the fermentation of dietary fibers by gut bacteria, mainly by Firmicutes (e.g., Faecalibacterium prausnitzii, Roseburia). They are crucial for maintaining gut health, providing energy to colonocytes, regulating immune responses, and maintaining the integrity of the gut barrier.
- IBS: In IBS patients, especially those with diarrhea-predominant IBS (IBS-D), there is often a reduced production of butyrate, which may contribute to increased gut permeability and visceral hypersensitivity. However, some studies have found increased levels of total SCFAs in the stool, possibly due to rapid transit time.
- IBD: In IBD, particularly in ulcerative colitis, there is a decrease in butyrate production due to the loss of butyrate-producing bacteria like Faecalibacterium prausnitzii. Reduced butyrate levels can lead to impaired epithelial barrier function, increased inflammation, and mucosal damage.
- Amino Acid Metabolites
- Metabolites: Indole, p-Cresol, Phenylacetate, Tryptamine
- Role in IBS and IBD:
- Gut bacteria metabolize amino acids such as tryptophan, tyrosine, and phenylalanine into various bioactive compounds.
- IBS: Tryptophan metabolites like indole and tryptamine can influence gut motility and serotonin production, which are often altered in IBS patients. For instance, altered levels of tryptamine are associated with changes in gut motility, and increased indole can enhance gut epithelial barrier function.
- IBD: In IBD, there is often an overproduction of metabolites like p-cresol, phenylacetate, and other phenolic compounds due to increased proteolytic fermentation. These metabolites can be toxic to the gut epithelium and exacerbate inflammation.
- Bile Acids
- Metabolites: Primary bile acids (Cholic acid, Chenodeoxycholic acid), Secondary bile acids (Deoxycholic acid, Lithocholic acid)
- Role in IBS and IBD:
- Bile acids are synthesized in the liver and modified by gut bacteria into secondary bile acids.
- IBS: In IBS-D, there is often an excess of bile acids in the colon due to malabsorption, leading to diarrhea and abdominal pain. Certain bacteria, such as Bacteroides and Clostridium, are involved in bile acid metabolism, and their altered abundance can affect bile acid levels.
- IBD: IBD patients often show altered bile acid metabolism, with a reduction in secondary bile acid levels due to a decrease in bile acid-transforming bacteria. This imbalance can contribute to gut dysbiosis and inflammation.
- Lipopolysaccharides (LPS)
- Metabolites: LPS
- Role in IBS and IBD:
- LPS are components of the outer membrane of Gram-negative bacteria and can trigger strong immune responses.
- IBS: Elevated LPS levels are associated with increased gut permeability (“leaky gut”) and low-grade inflammation, which are common in IBS patients.
- IBD: In IBD, increased LPS levels are linked to a heightened immune response, chronic inflammation, and damage to the intestinal epithelium.
- Gas Metabolites
- Metabolites: Methane, Hydrogen, Hydrogen Sulfide
- Role in IBS and IBD:
- These gases are produced by the fermentation of carbohydrates by gut bacteria.
- IBS: Increased methane production, often by Methanobrevibacter smithii, is associated with constipation-predominant IBS (IBS-C) due to its effect on slowing gut motility. Hydrogen and hydrogen sulfide can contribute to bloating, discomfort, and visceral pain.
- IBD: In IBD, the production of hydrogen sulfide, mainly by sulfate-reducing bacteria like Desulfovibrio, can impair epithelial cell metabolism and exacerbate mucosal inflammation.
- Polyamines
- Metabolites: Putrescine, Spermidine, Spermine
- Role in IBS and IBD:
- Polyamines are produced by gut bacteria and play a role in cell growth, proliferation, and inflammation.
- IBS: Altered levels of polyamines have been observed in IBS patients and may be linked to visceral sensitivity and epithelial cell turnover.
- IBD: Elevated polyamine levels are found in inflamed tissues of IBD patients and are thought to promote epithelial cell proliferation and immune responses, potentially contributing to disease pathology.
- Vitamins and Cofactors
- Metabolites: Vitamin K, B Vitamins (e.g., B12, B6, Folate)
- Role in IBS and IBD:
- Gut bacteria synthesize several essential vitamins and cofactors.
- IBS: Changes in gut microbiota composition in IBS can affect the production and absorption of these vitamins, potentially impacting overall health.
- IBD: In IBD, particularly in cases with extensive intestinal inflammation or resection, the synthesis and absorption of vitamins like B12 and folate can be significantly reduced, contributing to deficiencies.
Summary of Metabolite Alterations
- IBS: Decreased butyrate, altered bile acids, increased methane (IBS-C) or SCFAs (IBS-D), increased tryptamine and indole.
- IBD: Decreased butyrate and secondary bile acids, increased toxic amino acid metabolites, elevated LPS, hydrogen sulfide, and polyamines.
Impact of Major Dysbiosis on Drug Efficacy: How Gut Microbiota Imbalance Alters Therapeutic Outcomes
Major dysbiosis, which refers to an imbalance in the gut microbiota, can significantly impact the efficacy of various types of medicines in the body. The gut microbiota plays a crucial role in drug metabolism, influencing both pharmacokinetics (how the body absorbs, distributes, metabolizes, and excretes drugs) and pharmacodynamics (how drugs exert their effects on the body). Here’s how major dysbiosis can affect drug efficacy:
- Altered Drug Metabolism:
- Dysbiosis can change the expression and activity of gut microbial enzymes that are involved in the metabolism of drugs. This alteration can lead to either increased or decreased drug metabolism, affecting the drug’s bioavailability and therapeutic effect.
- For example, some gut bacteria can activate prodrugs (inactive compounds that become active in the body), and dysbiosis might impair this activation process, rendering the drug less effective.
- Impaired Drug Absorption:
- Gut microbiota influence the integrity of the intestinal barrier. Dysbiosis can damage this barrier, altering the absorption of orally administered drugs and nutrients. Reduced absorption can lead to sub-therapeutic drug levels, making treatments less effective.
- Increased Drug Toxicity:
- Dysbiosis may alter the metabolism of drugs in a way that produces harmful metabolites or increases the concentration of the active drug, leading to potential toxicity.
- For instance, drugs like irinotecan (used in cancer therapy) can be metabolized into toxic compounds by certain gut bacteria, which could worsen if dysbiosis is present.
- Impact on Drug Transporters and Receptors:
- Dysbiosis can modulate the expression of transporters and receptors in the gut, which play roles in drug uptake and response. Changes in these proteins can affect how well drugs are absorbed and how they exert their effects.
- Interaction with Immune System:
- The gut microbiota interacts closely with the immune system, and dysbiosis can lead to chronic inflammation or immune dysregulation. This altered immune state can affect the efficacy of drugs, particularly those that target the immune system, such as biologics and immunosuppressants.
- Impact on Personalized Medicine:
- Dysbiosis may affect how individuals respond to drugs that are typically tailored to their specific genetic or metabolic profiles, as gut microbiota contributes significantly to these profiles. Personalized treatments might become less predictable due to microbiota-driven variability.
- Influence on Drug Resistance:
- Gut microbiota can influence the development of drug resistance, especially in antibiotics. Dysbiosis can promote the growth of resistant bacteria, making antibiotic treatments less effective and contributing to the spread of resistant strains
Why Choose Novelgene:
- Unique In-house Protocols: Novelgene has developed proprietary methodologies for gut microbiota analysis, starting from stool sample collection to final analysis.
- Careful Sample Collection: Extra care is taken during stool sample collection, with controlled temperature management and transportation in specially designed boxes.
- Temperature Control & Processing: Ambient temperature is maintained during transportation, and samples are immediately processed for DNA isolation, preserving natural microbial diversity.
- Optimized Procedures: The entire procedure, from DNA isolation to sequencing, has been optimized for speed, making it faster than other labs in India.
- Advanced Markers and Tools: Novelgene has developed specialized markers and tools to identify potential metabolites produced by the microbial community and analyze microbiota ratios, which may be linked to neurological disorders.
- Comprehensive Microbiota Reports: 2-3 week
- GUT-Microbiome Diversity Score
- F/B Ratio: Firmicutes and Bacteroidetes are two major classes of Bacteria, dominant in the human gut microbiome. The Firmicutes to Bacteroidetes ratio (F/B ) correlates with the balance or imbalance in the gut microbiome. An ideal F/B ratio would lie between 0.5 to 1.2. An imbalance may indicate a risk for metabolic disorders.
- Know your most abundant bacteria, Pathogenic bacteria, opportunistic bacteria, probiotic bacteria etc with their %abundance
- Microbiome-based metabolic indicators: Butyrate, Gas, inflammatory and non-inflammatory producers.
- Finding on the basis of DYSBIOSIS and Summary Reports.
- Phase I: GUT-REBOOT (Initially 3-4 weeks under the supervision of gastroenterologist and nutritionist)
- Phase 2: GUT-REBUILD (Next 3-4 weeks under the supervision of gastroenterologist and nutritionist)
- Phase 3: GUT-RETAIN (Next 4-6 weeks under the supervision of gastroenterologist and nutritionist)
Samples Requirement: STOOL SAMPLE ONLY (samples collection will be taken care by Novelgene Wellness Team only)


