Neurological Disorder Test
Gut Microbiota & Metabolomics test for neurodegenerative disease
The gut microbiota plays a significant role in neurodegenerative diseases like Alzheimer’s and Parkinson’s through the gut-brain axis, influencing brain function, immune response, and neuroinflammation. Here’s an overview of how gut microbiota affects these diseases:
Microbiota Imbalance (Dysbiosis) in AD:
Studies show that patients with AD often have altered gut microbiota, characterized by increased levels of pro-inflammatory bacteria (e.g., Proteobacteria) and reduced levels of beneficial bacteria (e.g., Firmicutes and Bacteroidetes).
Dysbiosis can contribute to systemic inflammation, which exacerbates brain inflammation and amyloid-beta accumulation, a hallmark of AD.
Leaky Gut and Blood-Brain Barrier Dysfunction:
Dysbiosis can lead to a leaky gut, allowing toxins and inflammatory molecules to enter the bloodstream and reach the brain, weakening the blood-brain barrier.
This increased permeability allows harmful substances to penetrate the brain, promoting neuroinflammation and cognitive decline.
Production of Short-Chain Fatty Acids (SCFAs):
Healthy gut microbiota produce SCFAs like butyrate, which have anti-inflammatory effects and support brain health.
In AD, a reduction in SCFAs can lead to increased neuroinflammation, synaptic dysfunction, and accelerated disease progression.
Amyloid Production:
Certain gut bacteria can produce amyloid-like proteins, which may trigger an immune response and contribute to amyloid-beta deposition in the brain.
- Results and Finding:
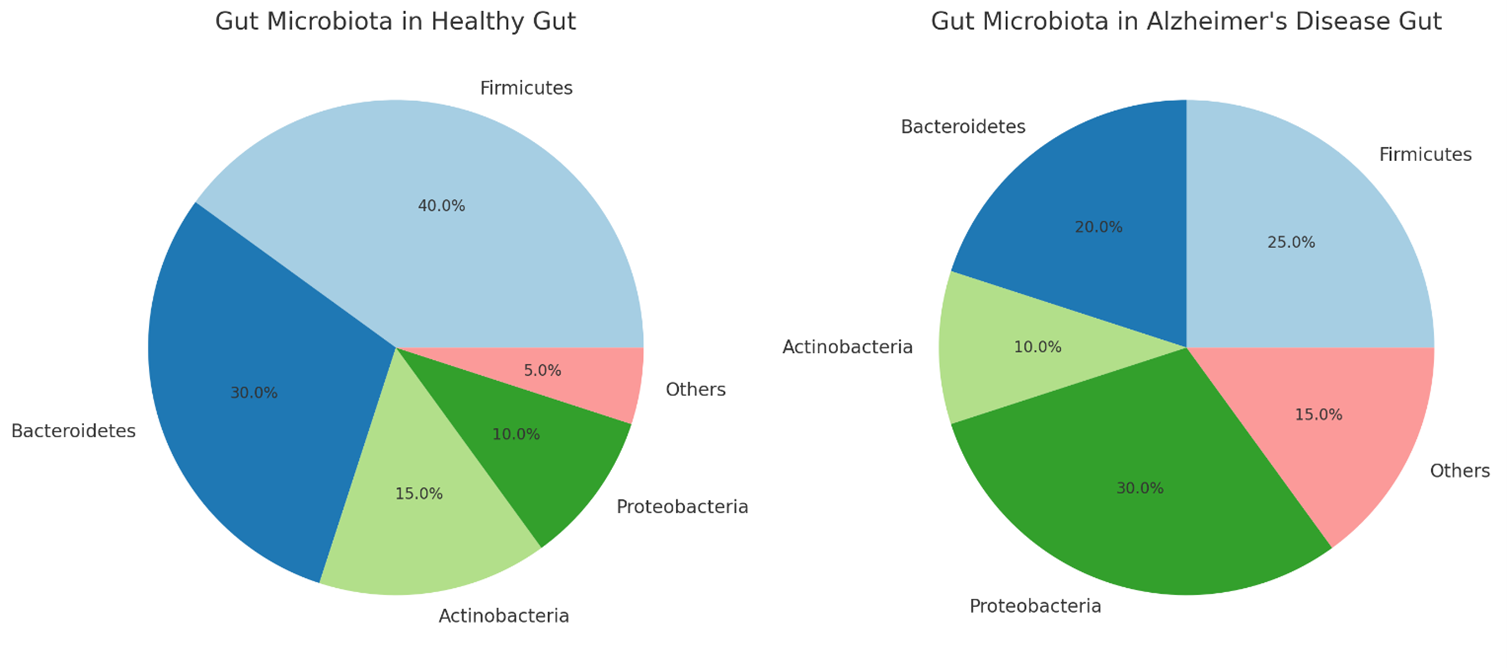
The pie charts above illustrate the gut microbiota composition in a healthy gut compared to an Alzheimer’s disease (AD) gut:
- Healthy Gut:
- Firmicutes (40%)
- Bacteroidetes (30%)
- Actinobacteria (15%)
- Proteobacteria (10%)
- Others (5%)
- Alzheimer’s Disease Gut:
- Firmicutes (25%)
- Bacteroidetes (20%)
- Actinobacteria (10%)
- Proteobacteria (30%)
- Others (15%)
In AD, there’s a noticeable increase in Proteobacteria and a decrease in Firmicutes and Bacteroidetes, reflecting dysbiosis associated with the disease
Impact of Major Dysbiosis on Drug Efficacy: How Gut Microbiota Imbalance Alters Therapeutic Outcomes
Major dysbiosis, which refers to an imbalance in the gut microbiota, can significantly impact the efficacy of various types of medicines in the body. The gut microbiota plays a crucial role in drug metabolism, influencing both pharmacokinetics (how the body absorbs, distributes, metabolizes, and excretes drugs) and pharmacodynamics (how drugs exert their effects on the body). Here’s how major dysbiosis can affect drug efficacy:
- Altered Drug Metabolism:
- Dysbiosis can change the expression and activity of gut microbial enzymes that are involved in the metabolism of drugs. This alteration can lead to either increased or decreased drug metabolism, affecting the drug’s bioavailability and therapeutic effect.
- For example, some gut bacteria can activate prodrugs (inactive compounds that become active in the body), and dysbiosis might impair this activation process, rendering the drug less effective.
- Impaired Drug Absorption:
- Gut microbiota influence the integrity of the intestinal barrier. Dysbiosis can damage this barrier, altering the absorption of orally administered drugs and nutrients. Reduced absorption can lead to sub-therapeutic drug levels, making treatments less effective.
- Increased Drug Toxicity:
- Dysbiosis may alter the metabolism of drugs in a way that produces harmful metabolites or increases the concentration of the active drug, leading to potential toxicity.
- For instance, drugs like irinotecan (used in cancer therapy) can be metabolized into toxic compounds by certain gut bacteria, which could worsen if dysbiosis is present.
- Impact on Drug Transporters and Receptors:
- Dysbiosis can modulate the expression of transporters and receptors in the gut, which play roles in drug uptake and response. Changes in these proteins can affect how well drugs are absorbed and how they exert their effects.
- Interaction with Immune System:
- The gut microbiota interacts closely with the immune system, and dysbiosis can lead to chronic inflammation or immune dysregulation. This altered immune state can affect the efficacy of drugs, particularly those that target the immune system, such as biologics and immunosuppressants.
- Impact on Personalized Medicine:
- Dysbiosis may affect how individuals respond to drugs that are typically tailored to their specific genetic or metabolic profiles, as gut microbiota contributes significantly to these profiles. Personalized treatments might become less predictable due to microbiota-driven variability.
- Influence on Drug Resistance:
- Gut microbiota can influence the development of drug resistance, especially in antibiotics. Dysbiosis can promote the growth of resistant bacteria, making antibiotic treatments less effective and contributing to the spread of resistant strains
Why Choose Novelgene:
- Unique In-house Protocols: Novelgene has developed proprietary methodologies for gut microbiota analysis, starting from stool sample collection to final analysis.
- Careful Sample Collection: Extra care is taken during stool sample collection, with controlled temperature management and transportation in specially designed boxes.
- Temperature Control & Processing: Ambient temperature is maintained during transportation, and samples are immediately processed for DNA isolation, preserving natural microbial diversity.
- Optimized Procedures: The entire procedure, from DNA isolation to sequencing, has been optimized for speed, making it faster than other labs in India.
- Advanced Markers and Tools: Novelgene has developed specialized markers and tools to identify potential metabolites produced by the microbial community and analyze microbiota ratios, which may be linked to neurological disorders.
- Comprehensive Microbiota Reports: 2-3 week
- GUT-Microbiome Diversity Score
- F/B Ratio: Firmicutes and Bacteroidetes are two major classes of Bacteria, dominant in the human gut microbiome. The Firmicutes to Bacteroidetes ratio (F/B ) correlates with the balance or imbalance in the gut microbiome. An ideal F/B ratio would lie between 0.5 to 1.2. An imbalance may indicate a risk for metabolic disorders.
- Know your most abundant bacteria, Pathogenic bacteria, opportunistic bacteria, probiotic bacteria etc with their %abundance
- Microbiome-based metabolic indicators: Butyrate, Gas, inflammatory and non-inflammatory producers.
- Finding on the basis of DYSBIOSIS and Summary Reports.
- Phase I: GUT-REBOOT (Initially 3-4 weeks under the supervision of gastroenterologist and nutritionist)
- Phase 2: GUT-REBUILD (Next 3-4 weeks under the supervision of gastroenterologist and nutritionist)
- Phase 3: GUT-RETAIN (Next 4-6 weeks under the supervision of gastroenterologist and nutritionist)
Approach and Solution for This DYSBIOSIS
Balancing dysbiosis and restoring a healthy gut microbiota in Alzheimer’s disease (AD) involves several strategies aimed at increasing beneficial bacteria like Firmicutes, Bacteroidetes, and Actinobacteria, while reducing harmful bacteria like Proteobacteria. Here are specific interventions to help restore gut microbiota balance:
- Diet Modification
- Increase Fiber Intake: A diet rich in dietary fiber promotes the growth of Firmicutes and Bacteroidetes, which are responsible for producing beneficial short-chain fatty acids (SCFAs) like butyrate.
- Sources: Whole grains, vegetables, fruits, legumes, and nuts.
- Effect: Fiber promotes the growth of beneficial bacteria and inhibits the overgrowth of Proteobacteria.
- Mediterranean Diet: This diet is rich in fruits, vegetables, whole grains, and healthy fats (e.g., olive oil) and has been associated with reduced risk of cognitive decline.
- It supports beneficial bacteria and helps reduce inflammatory species like Proteobacteria.
- Polyphenol-Rich Foods: Foods like berries, green tea, and dark chocolate are high in polyphenols, which act as prebiotics and promote the growth of Bacteroidetes and Actinobacteria.
- Probiotics
- Supplementation with Beneficial Bacteria: Probiotics can help restore the balance of gut bacteria by introducing live beneficial bacteria.
- Lactobacillus and Bifidobacterium species are known to increase the abundance of Firmicutes and reduce harmful bacteria like Proteobacteria.
- Specific Probiotic Strains:
- Lactobacillus rhamnosus and Bifidobacterium longum promote anti-inflammatory responses and support brain function.
- Multi-strain Probiotics: Can promote the growth of beneficial Firmicutes and reduce dysbiosis.
- Prebiotics
- Prebiotic Fibers: These are non-digestible carbohydrates that feed beneficial bacteria, particularly Firmicutes and Bacteroidetes.
- Inulin: Found in chicory root, onions, garlic, and bananas, it specifically enhances Bifidobacteria and Firmicutes
- Fructooligosaccharides (FOS) and Galactooligosaccharides (GOS): These prebiotics encourage the growth of beneficial microbes like Bacteroidetes and inhibit pathogenic bacteria like Proteobacteria.
- Fecal Microbiota Transplantation (FMT)
- Restoration of Healthy Microbiota: FMT involves transferring stool from a healthy donor into the gut of a person with dysbiosis to restore a balanced microbial environment.
- Studies have shown FMT to improve cognitive functions and reduce neuroinflammation by correcting microbiota imbalances in AD models.
- Reduce Intake of Pro-Inflammatory Foods
- Avoid Processed Foods and Sugars: Diets high in sugar and processed foods promote the growth of harmful Proteobacteria and reduce the diversity of beneficial bacteria.
- Minimize Saturated Fats and Red Meat: These can lead to increased Proteobacteria and gut inflammation, which is linked to cognitive decline.
- Omega-3 Fatty Acids
- Anti-inflammatory Effects: Omega-3 fatty acids (found in fatty fish, flaxseeds, and walnuts) have been shown to promote the growth of Bacteroidetes and Firmicutes and reduce the abundance of inflammatory bacteria.
- Omega-3s also have neuroprotective properties, helping to mitigate neuroinflammation associated with AD.
- Polyphenol Supplementation
- Polyphenols from sources like green tea, curcumin (from turmeric), and resveratrol (from red grapes) can promote the growth of Actinobacteria and Bacteroidetes.
- These compounds also have strong anti-inflammatory effects, reducing the burden of Proteobacteria.
- Antibiotics (Cautiously Applied)
- In cases of severe dysbiosis, antibiotics may be used to reduce the overgrowth of harmful bacteria (Proteobacteria), but they must be used cautiously as they can also harm beneficial bacteria.
- Follow-up with prebiotics and probiotics is essential to re-establish a healthy microbiota after antibiotic treatment.
- Physical Activity
- Regular exercise has been linked to an increase in microbial diversity, with beneficial effects on Firmicutes and Bacteroidetes.
- Exercise can reduce systemic inflammation and support gut health, indirectly improving brain health.
- Stress Reduction
- Chronic stress can exacerbate gut dysbiosis by promoting the growth of harmful bacteria like Proteobacteria.
- Mindfulness, meditation, and other stress-relief techniques may help maintain a healthy gut-brain axis.
Summary of Actions to Balance Gut Microbiota in AD:
- Increase beneficial bacteria (Firmicutes, Bacteroidetes, and Actinobacteria) through a fiber-rich, polyphenol-heavy, and prebiotic diet.
- Reduce harmful bacteria (Proteobacteria) by avoiding processed foods, reducing saturated fats, and incorporating omega-3s.
- Introduce beneficial microbes via probiotics or FMT, and support their growth with prebiotics.
- Address lifestyle factors like stress and inactivity to further support a healthy microbiota.
By combining these dietary, probiotic, and lifestyle interventions, it may be possible to correct the dysbiosis observed in Alzheimer’s disease and promote a healthier gut-brain axis.


